Any Mountain Expedition Team
The Any Mountain project is dedicated to funding research on the prevention and early detection of ovarian cancer. This research is being conducted by an incredible team of gynecologic oncology leaders from around the country.
We are creating the first international surgical ovarian cancer prevention trial which could revolutionize how women choose to decrease their risk of ovarian cancer. Next up: novel efforts to improve early detection, treatment and prevention.
Any Mountain is expressly committed to ending racism within healthcare and beyond. Research priority will be given to projects addressing intersections of racial justice and ovarian cancer. Ending racism saves lives.
Dr. Elizabeth Swisher

Co-Leader Breast and Ovarian Cancer Program, Fred Hutchinson/University of Washington Cancer Consortium
Read moreDr. Elizabeth Swisher
Dr. Elizabeth Swisher has extensive research experience in the genetics of ovarian cancer. She worked with the geneticist who discovered the BRCA1 gene, Dr. Mary-Claire King, for more than 20 years. Dr. Swisher has contributed to the scientific community’s understanding of which genes are responsible for hereditary ovarian cancer. She also established and ran a multidisciplinary, high-risk prevention clinic for women at high risk of breast and ovarian cancer.
Dr. Swisher is involved in the Any Mountain Project because she is passionate about raising awareness for the genetic risk of ovarian cancer and about preventing all hereditary ovarian cancer. “Every woman who dies of hereditary ovarian cancer is a life wasted needlessly,” she says.
Dr. Swisher received her undergraduate degree from Yale University in 1987. She did her graduate work at the University of California San Diego School of Medicine from 1988-1992. Prior to becoming a professor at the University of Washington School of Medicine, she interned there from 1992-1993 and completed her residency there between 1993 and 1996. She also completed a fellowship in the Washington University Division of Gynecologic Oncology, Department of Obstetrics and Gynecology from 1996-1999.
Dr. Swisher enjoys hiking, golfing, skiing, snowshoeing, playing soccer, traveling, reading, and in general, enjoying the outdoors with her family and her dogs. She embodies everything that it means to be a part of the Any Mountain Expedition Team.

Dr. Karen Lu

Chair, Department of Gynecologic Oncology and Reproductive Medicine, J. Taylor Wharton Distinguished Chair, The University of Texas MD Anderson Cancer Center
Read moreDr. Karen Lu
Dr. Karen Lu’s career has focused on ovarian cancer prevention and early detection for the last 20 years. She is the Chair of the Department of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center.
Dr. Lu attended medical school at Yale University and completed her residency at Harvard University through the Brigham and Women’s Hospital and Massachusetts General Hospital combined program. She also completed a fellowship through Harvard University and the Dana-Farber/Brigham and Women’s Cancer Center.
Dr. Lu is passionate about research in ovarian cancer prevention and early detection. She is excited to partner with a group that shares her passion for developing patient-centered ovarian cancer prevention strategies through Any Mountain. “We are identifying a large cohort of healthy women who are told they are at substantially increased risk for ovarian cancer due to a genetic mutation,” she says. “Let’s work across the world to conduct clinical trials to develop ways to prevent it or catch it early.”
Dr. Lu enjoys being active with her family and she especially enjoys hiking in the U.S. and around the world. We are delighted to have her on the Any Mountain Expedition Team.

Dr. Jamie Bakkum-Gamez

Professor of OB/GYN, Consultant in Gynecologic Oncology Surgery, Mayo Clinic, Rochester, Minnesota
Read moreDr. Jamie Bakkum-Gamez
Dr. Jamie Bakkum-Gamez has been a practicing gynecologic oncologist on staff at the Mayo Clinic for the past 10 years. Since joining the staff in 2009, she has lead an active, multidisciplinary research team that is studying unique DNA markers that occur in ovarian cancer, endometrial cancer, and cervical cancer with the goal of developing an earlier detection test for ovarian and endometrial cancer. Dr. Bakkum-Gamez is the Mayo Clinic site PI for the WISP trial and she was a co-author of the ovarian cancer risk reduction (RRSO) procedure that was added to the National Comprehensive Cancer Network (NCCN) guidelines in 2016.
As a gynecologic oncologist, Dr. Bakkum-Gamez cares for women with advanced stage ovarian cancer and improves survival from this disease. She says that in her experience, ovarian cancer “needs better treatments, effective early detection, and optimized risk reduction strategies.” She says this is critical, which is why she is involved in the Any Mountain Project.
At the Mayo Clinic, Dr. Bakkum-Gamez cares for women with and at risk for gynecologic malignancies and she designs and/or runs clinical trials for early gynecologic cancer detection, cancer risk reduction, and novel therapies for gynecologic cancer. Her clinical practice is solely surgical, providing surgery to women with ovarian cancer as well as women with family histories or known mutations that elevate their risks for ovarian cancer. Dr. Bakkum-Gamez attended the University of Wisconsin Medical School and she completed her OB/GYN residency at the Mayo Clinic. She completed a Gynecologic Oncology Fellowship there as well.
In her free time, Dr. Bakkum-Gamez gardens and grows Shiitake mushrooms. She loves to run, watch scary movies and Indie flicks, and she has become an avid fan of podcasts in the past few years. We are honored for Dr. Bakkum-Gamez to be a part of the Any Mountain Expedition Team.

Dr. Stephanie Blank

Professor of Obstetrics, Gynecology, and Reproductive Science, Icahn School of Medicine at Mount Sinai; Director of Gynecologic Oncology, Mount Sinai Health System; Director of Oncology Programs, Blavatnik Family Women’s Health Research Institute; Director of Women’s Health, Blavatnik Family Chelsea Medical Center at Mount Sinai
Read moreDr. Stephanie Blank
Dr. Stephanie Blank teaches obstetrics, gynecology, and reproductive science to students of the Icahn School of Medicine at Mount Sinai. She is also the director of many of their women’s health and gynecologic oncology programs. In her role, she cares for many women with ovarian cancer.
Dr. Blank believes that, learning about cancer risk and sharing this information with family members who can actually act on it is empowering for women with ovarian cancer, and the message that this cancer can be prevented, is compelling and inspiring at a time when these women might not feel so hopeful. “Prevention works,” she says. “The incidence of ovarian cancer is decreasing due to prevention efforts. The Any Mountain Expedition Team is poised to make a difference. Countering cancer by raising awareness through creativity and educating people to take charge of their genetic cancer risk is a challenge – a true mountain – but this amazing group can do it. Any Mountain stresses teamwork, creativity, collaboration, and progress.”
Dr. Blank attended medical school at the University of California San Diego School of Medicine. She completed her residency at the Weill Cornell School of Medicine and she completed a fellowship at the University of Pennsylvania.
Dr. Blank enjoys laughing, hiking, running, swimming in the ocean, mountains, traveling, reading, and being outside as much as possible. We are grateful for her contributions to the Any Mountain Expedition Team.

Dr. Dineo Khabele

Chair of the Department of Obstetrics and Gynecology, Mitchell and Elaine Yanow Professor of Obstetrics and Gynecology, Washington University School of Medicine in St. Louis
Read moreDr. Dineo Khabele
Dr. Dineo Khabele is a fierce advocate for advancing ovarian cancer research and care. She helped develop a multidisciplinary high-risk breast and ovarian cancer clinic at the University of Kansas Cancer Center and her research has focused on ovarian cancer and novel therapeutic strategies.
Dr. Khabele’s most recent studies have provided the basis for a clinical trial for chemotherapy-resistant ovarian cancer. Her work has been funded by the National Institutes of Health (NIH) including two current awards and a new award just being issued. Her research has been recognized by many awards and elections, including most recently election to the American Society for Clinical Investigation in 2019.
Dr. Khabele did her undergraduate and medical school studies at Columbia University. After residency in Obstetrics and Gynecology at New York Presbyterian Hospital/Cornell University Medical Center, she completed a fellowship in Gynecologic Oncology at Albert Einstein College of Medicine and Montefiore Medical Center. After four years as an Assistant Professor in the Department of Obstetrics and Gynecology at Meharry Medical College, she joined the faculty of Vanderbilt University where she spent the next nine years holding appointments in both the Department of Obstetrics and Gynecology and the Department of Cancer Biology and served as the Director of Gynecologic Oncology Translational Research. She recently started a gynecologic oncology fellowship program at University of Kansas Medical Center and she is very involved in many national and local committees and volunteer programs.
Dr. Khabele likes to run, read, and relax on the beach or at a spa. We are proud to have such an accomplished doctor on the Any Mountain Expedition Team.

Dr. Kara Long Roche

Assistant Attending, Gynecologic Surgery, Associate Director, Gynecologic Oncology Fellowship Program, Section of Ovarian Cancer Surgery, Department of Surgery, Memorial Sloan Kettering Cancer Center
Read moreDr. Kara Long Roche
Dr. Kara Long Roche is a surgeon who cares for women with gynecologic cancer and a researcher focusing on prevention, early detection, and the surgical management of ovarian cancer. In addition to her training and years of experience caring for women at risk for and with ovarian cancer, she herself is the daughter of an ovarian cancer survivor.
“Experiencing the disease from the perspective of a patient’s family provided me with a deeper understanding of the impact this disease can have on a woman and her family,” she says. “Years after my mother was diagnosed, we learned that she carried a genetic mutation – a mutation that I share with her and one which has empowered me to make decisions to protect myself from developing this disease. Any Mountain is a team of strong, intelligent, and empowered physicians who share my passion for preventing ovarian cancer. Coming together allows us to reach more women and prevent more cases of this terrible disease.”
Dr. Long Roche attended medical school at the University of North Carolina at Chapel Hill. She received her Master’s in Health Science Research from Weill Cornell and she completed her OB/GYN residency at the New York University Medical Center. She completed a fellowship in Gynecologic Oncology at Memorial Sloan Kettering Cancer Center, where she works today.
Dr. Long Roche likes to spend her free time with family and friends and she enjoys playing outside with her kids. One of her all-time favorite activities is running. We appreciate the heart and experiences that Dr. Long Roche brings to the Any Mountain project.

How the Dollars Raised for the Everest Trip are Being Used
Together, we raised over $500,000 for ovarian cancer awareness, prevention, and early detection research.
More than 700 people donated between $10 – $75,000 dollars
Over
$500,000
total raised
After expenses…
$300,00
to Research
$75,000
to Creative Awareness
$25,000
to Project Administration
The research money raised went to the Any Mountain Expedition research team lead by Dr. Elizabeth Swisher from University of Washington and Dr. Karen Lu from MD Anderson. The allocation of research funds is being overseen the Minnesota Ovarian Cancer Alliance under the leadership of their Executive Director Kathleen Gavin.
TUBA-WISP II Trial
MOCA is distributing funds raised by the Any Mountain trek to Mt. Everest to cancer centers throughout the U.S. to support the expansion of the international TUBA-WISP II trial. This surgical prevention trial, managed by MD Anderson Cancer Center, gives women, who are at high risk for ovarian cancer due to genetic mutations, the option of a two-step procedure to reduce their risk. By having their fallopian tubes surgically removed first (salpingectomy) with a delayed ovary removal (oophorectomy), women are able to preserve hormonal function longer, thereby maintaining cardiovascular protection, sexual function and other benefits.
The Any Mountain funding is being used to open sites across the country expanding access to diverse populations.
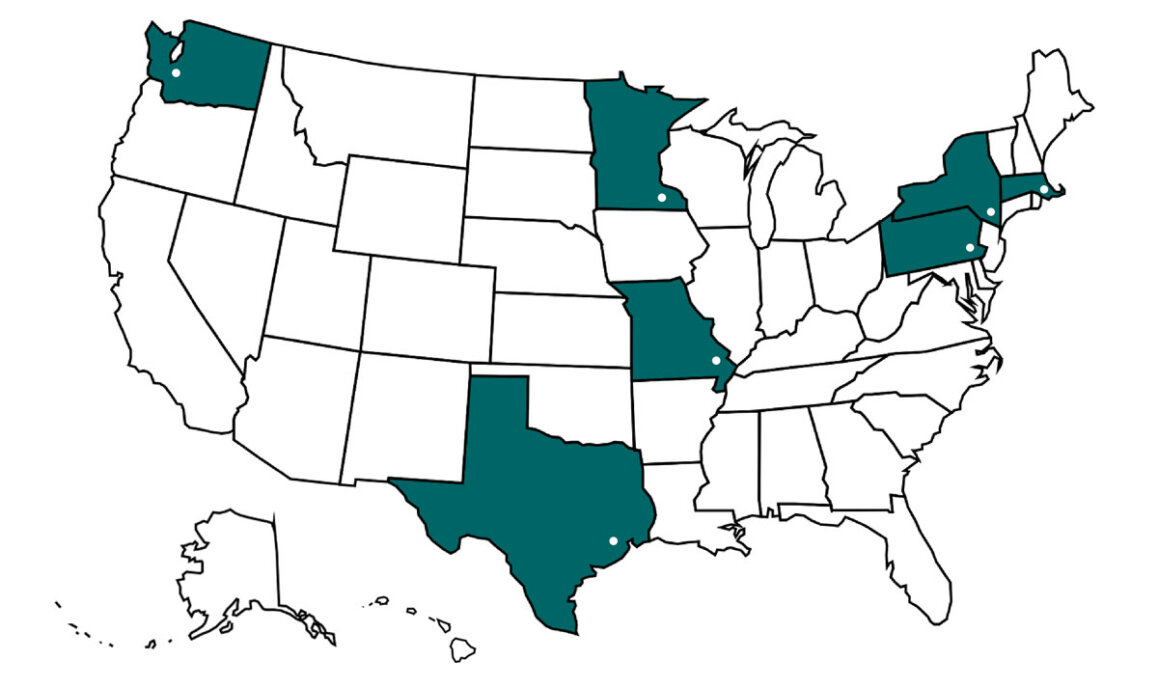
The Any Mountain TUBA-WISP II trial is currently open and enrolling patients at:
- MD Anderson (Houston, Texas)
- Mayo Clinic (Rochester, MN)
- Memorial Sloan Kettering (New York City)
- University of Washington (Seattle)
- Dana Farber Cancer Institute (Boston)
- Wahington University (St. Louis)
- Mount Sinai (NYC)
- University of Pennsylvania (Philadelphia)


For more information about the trial:
“My love you were with me, right by my side. Acting really tough, trying hard not to cry. You said to be strong, we’ll fight this head on. Take a leap of faith, and the journey’s begun.”
Our
Research
The Any Mountain project is made possible by Let Every Woman Know – Alaska and the Minnesota Ovarian Cancer Alliance.
Let Every Woman Know – Alaska
Let Every Woman Know – Alaska is dedicated to raising awareness, sharing information and saving lives by educating people in the Last Frontier and supporting women living with gynecologic cancers. It’s an Alaska-based non-profit, founded by Dr. Joanie Hope, which provides creative state-wide education, support, advocacy, and arts programs unique to the state’sdiverse communities.
Minnesota Ovarian Cancer Alliance
The Minnesota Ovarian Cancer Alliance (MOCA) is one of our key partners and committed to responsibly managing the funds raised for ovarian cancer research through the Any Mountain project. MOCA is a 501(c)3 non-profit organization dedicated to funding ovarian cancer research, providing support to the women and families affected by ovarian cancer, educating the medical community, and raising awareness of ovarian cancer.
“Been two years now and I’m still cancer free. I know I should be happy, but I don’t feel at ease. I need a vacation, from my life as a patient. Refuge from the steady blast of cancer’s damnation.”
Any Mountain Research Awards
October 2021
The following research projects were approved for one year of funding by Any Mountain in October 2021 and are currently underway.
TUBA-WISP II Study
$65,000
Principal Investigator: Karen Lu, MD at MD Anderson, Houston, TX
The TUBA-WISP II Study is a world-wide prevention study in high-risk women with multiple centers in the United States. This trial is designed to determine whether removing the fallopian tubes and delaying ovarian removal by 5 years is effective for reducing hereditary ovarian cancer risk, which would allow women to keep their ovarian function intact for a longer period of time. Women self-select whether they want to undergo a standard removal of ovaries and fallopian tubes in one surgery or a new option to remove only the fallopian tubes and some years later remove the ovaries. Any Mountain funds will be used to finalize data forms, set up the national database, assist sites with IRB submissions and begin patient enrollment. Data from similar studies being done in other countries around the world will be pooled together to determine how safe and effective the new prevention option is for high risk women. Data from this trial could change the standard care for women at high risk of ovarian cancer
Understanding Decision Making for Risk Reducing Surgery
$30,000
Principal Investigator: Kara Long Roche, MD at Memorial Sloan Kettering, NYC
This study will identify common themes and influential factors in the decision to undergo risk-reducing removal of the tubes and ovaries in young women at high genetic risk of developing ovarian cancer. The researchers will determine sociodemographic, medical, and psychosocial factors that contribute to the decision-making process. Data from this study will help us better counsel women about options for managing an increased risk of ovarian cancer.
Predictor of Completion of Genetic Testing: An Ancillary Analysis of the MAGENTA Study
$30,000
Principal Investigator: Barbara Norquist, MD at University of Washington, Seattle
THE MAGENTA study was a large trial that evaluated how much genetic counseling was needed to deliver testing for cancer genetic risk to women in their homes. This ancillary analysis of the MAGENTA study will help us better understand why some people start the process, but then do not complete genetic testing at home by interviewing women who did and did not complete the testing process. Results from this study will help us identify concerns and barriers to genetic testing for cancer risk and inform future interventions to bring risk assessment to more people.





